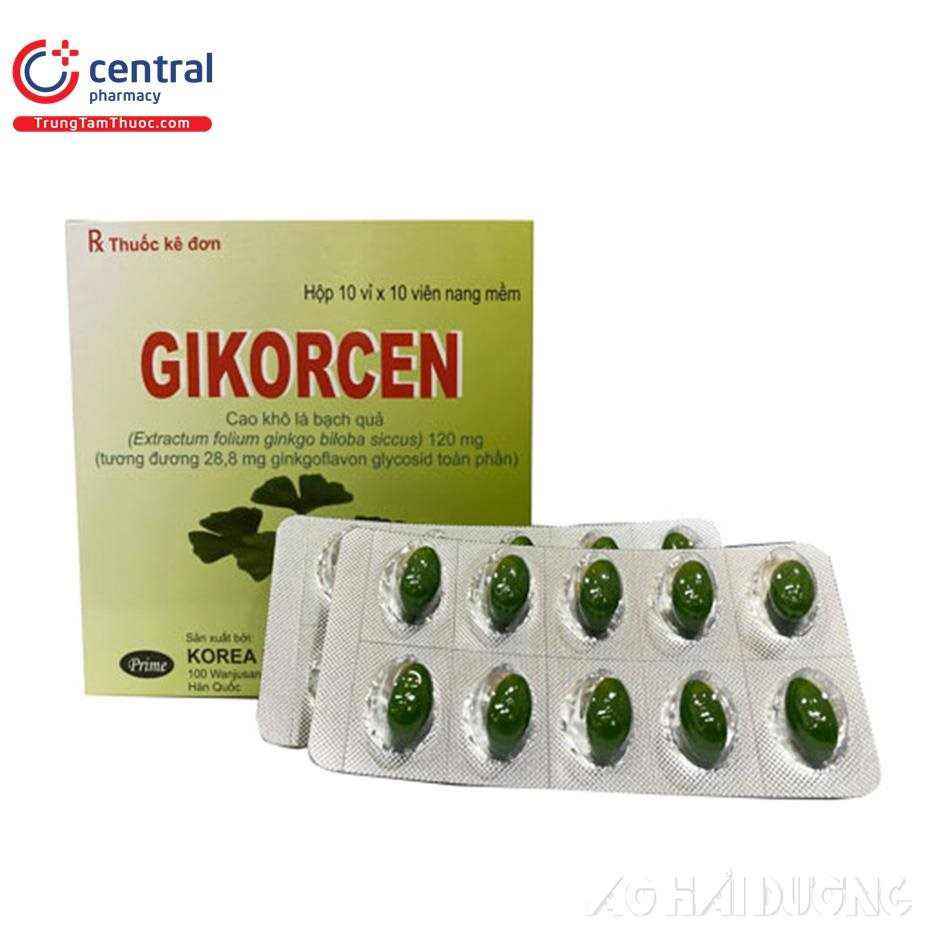
Gikorcen soft capsule samples do not meet quality standards and must be recalled to ensure user safety.
On September 18, Hai Duong Department of Health requested PEM Pharmaceutical Company Limited (No. 4B/48 Nguyen Thuong Man, Binh Han Ward, Hai Duong City) to coordinate with business establishments, users that have been distributed, manufacturing establishments (including establishments not located in Hai Duong province) and related units to recall all Gikorcen soft capsules; send the recall report to the Department of Drug Administration (Ministry of Health) and the Department of Health within 18 days.
The District Health Department shall notify all medical facilities, drug trading and using establishments in the area under its management of the content of the above capsule recall. Organize inspection and supervision of medical facilities, drug trading and using establishments under its management. If any batch of drugs is found to not meet the quality standards stated in Official Dispatch No. 9435/QLD-CL dated September 15, 2023 of the Department of Drug Administration, it must organize the recall, handle and report according to regulations to the Department of Health.
Medical units and drug businesses in the province must review the entire list of drugs being used and traded at the unit and its affiliated units. If any batch of drugs is found to not meet the above quality standards, it must be recalled and handled according to regulations.
The Department requests that its affiliated units coordinate to widely propagate so that people know and do not trade or use the above batch of drugs; organize inspections and supervise the compliance with regulations of PEM Pharmaceutical Company Limited...
Gikorcen soft capsules are manufactured by Korea Prime Pharm. Co., Ltd. (Korea), imported by Delta Medical Import-Export Co., Ltd. (registration number VN-22803-21, batch number 5662212, expiry date August 29, 2025), and distributed by PEM Pharmaceutical Co., Ltd. This is a drug indicated for the treatment of cognitive dysfunction, restoration of the degenerative nervous system, enhancement of alertness and clarity, and improvement of cognitive decline and neurological disorders.
On September 15, 2023, the Department of Drug Administration - Ministry of Health announced that the Gikorcen soft capsule sample did not meet quality standards and requested a recall.
PV